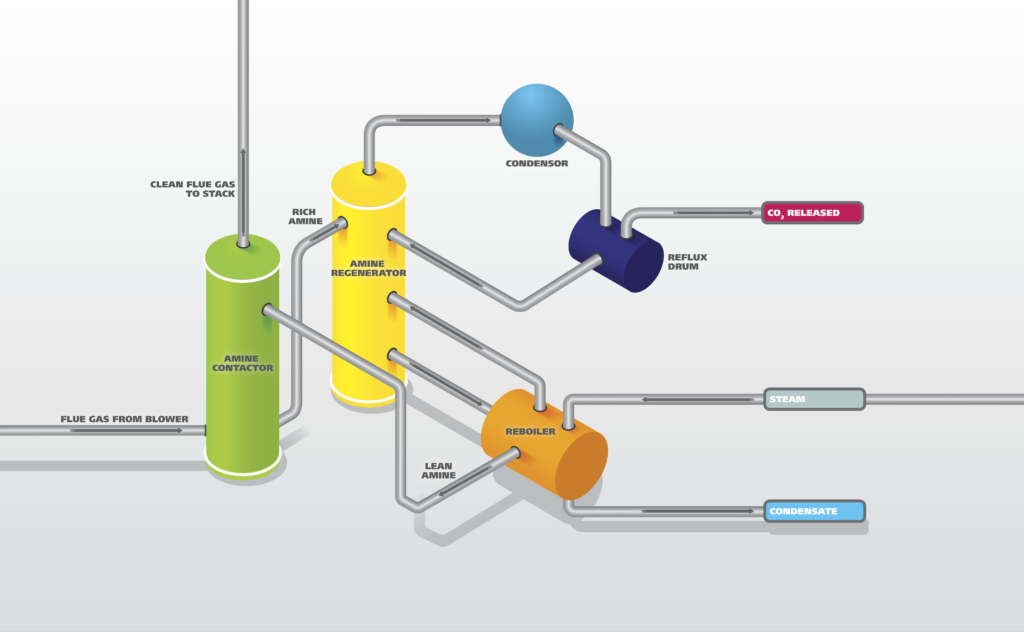
Amine Treatment
An amine treating unit captures hydrogen sulfide and other acidic gases from the refinery gas streams and concentrates them into an amine solution. It is also used to capture acidic gases from raw or sour natural gasses. Amine gas sweetening is a proven technology that removes H₂S and CO₂ from natural gas and liquid hydrocarbon streams through absorption and chemical reaction. The entire process is very energy intensive and results in high operating costs. Optimizing the amine activity and usage by online analysis is a critical step in reducing overall costs and measuring the efficiency of the CO₂ capture at the same time
Online Monitoring of Amine Strength
Determination of free amine is an important parameter to ensure acid gas removal. The alkalinity of gas washing solutions containing alkanolamines is measured by potentiometric titration with sulphuric acid using a combined glass electrode.
A single online analyzer can monitor several sample streams and determine the binding capacity of several amine scrubbers in succession. By implementing Metrohm’s fully automated online monitoring solution for this process, it is possible to optimize the amine activity and measure the efficiency of the acidic gas capture, reducing overall costs while ensuring environmental compliance.
Monitoring Heat Stable Salt Formation by Ion Chromatography
Heat stable salts are a product of the neutralization reaction between the alkaline amine and an organic or inorganic acid (the neutralizing agent). Amine solutions extract other contaminants that form salts of organic acids and sulfur species such as oxalic acid, formic acid, thiosulfate and thiocyanate. The accumulation of heat-stable salts not only causes a reduction in CO₂ absorption capacity, but also causes a significant increase in the system corrosiveness. Metrohm provides a simple to use Isocractic IC method to determine heat stable salts.
Bicine Determination by Ion Chromatography
During degradation of these amines they form various products, especially bicine which is corrosive. It is extremely important to determine the accurate amount of bicine in order to control corrosion and cost. Bicine can be determined using cation exchange chromatography with electrochemical detection.
